In this article, You will read Origin of Atmosphere – for UPSC IAS (Geography)
Other planets and moons in our solar system have atmospheres, but none of them could support life as we know it. They are either too dense (as on Venus) or not dense enough (as on Mars), and none of them have much oxygen, the precious gas that we Earth animals need every minute.
So how did our atmosphere get to be so special?
Some scientists describe three stages in the evolution of Earth’s atmosphere as it is today
Origin of Atmosphere
- Earth is believed to have formed about 5 billion years ago.
- In the first 500 million years, a dense atmosphere emerged from the vapor and gases that were expelled during degassing of the planet’s interior.
- During the cooling of the earth, gases and water vapour were released from the interior solid earth. This started the evolution of the present atmosphere. This process is called degassing.
- These gases may have consisted of hydrogen (H2), water vapor, methane (CH4) , and carbon oxides.
- Prior to 3.5 billion years ago the atmosphere probably consisted of carbon dioxide (CO2), carbon monoxide (CO), water (H2O), nitrogen (N2), and hydrogen.
- The hydrosphere was formed 4 billion years ago from the condensation of water vapor, resulting in oceans of water in which sedimentation occurred.
- The most important feature of the ancient environment was the absence of free oxygen. Evidence of such an anaerobic reducing atmosphere is hidden in early rock formations that contain many elements, such as iron and uranium, in their reduced states. Elements in this state are not found in the rocks of mid-Precambrian and younger ages, less than 3 billion years old.
- One billion years ago, early aquatic organisms called blue-green algae began using energy from the Sun to split molecules of H2O and CO2 and recombine them into organic compounds and molecular oxygen (O2). This solar energy conversion process is known as photosynthesis. Some of the photosynthetically created oxygen combined with organic carbon to recreate CO2 molecules. The remaining oxygen accumulated in the atmosphere, touching off a massive ecological disaster with respect to early existing anaerobic organisms. As oxygen in the atmosphere increased, CO2 decreased.
- High in the atmosphere, some oxygen (O2) molecules absorbed energy from the Sun’s ultraviolet (UV) rays and split to form single oxygen atoms. These atoms combining with remaining oxygen (O2) to form ozone (O3) molecules, which are very effective at absorbing UV rays. The thin layer of ozone that surrounds Earth acts as a shield, protecting the planet from irradiation by UV light.
- The amount of ozone required to shield Earth from biologically lethal UV radiation, wavelengths from 200 to 300 nanometers (nm), is believed to have been in existence 600 million years ago. At this time, the oxygen level was approximately 10% of its present atmospheric concentration.
- Prior to this period, life was restricted to the ocean. The presence of ozone enabled organisms to develop and live on the land. Ozone played a significant role in the evolution of life on Earth and allows life as we presently know it to exist.
3 Phases of Atmosphere Formation
- Just formed Earth: Like Earth, the hydrogen (H2) and helium (He) was very warm. These molecules of gas moved so fast they escaped Earth’s gravity and eventually all drifted off into space.
- Earth’s original atmosphere was probably just hydrogen and helium because these were the main gases in the dusty, gassy disk around the Sun from which the planets formed. The Earth and its atmosphere were very hot. Molecules of hydrogen and helium move really fast, especially when warm. Actually, they moved so fast they eventually all escaped Earth’s gravity and drifted off into space.

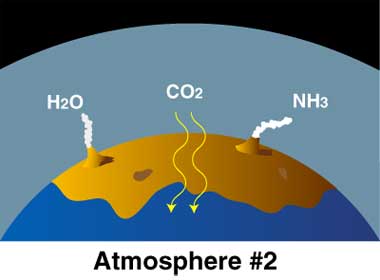
- Young Earth: Volcanoes released gases H2O (water) as steam, carbon dioxide (CO2), and ammonia (NH3). Carbon dioxide dissolved in seawater. Simple bacteria thrived on sunlight and CO2. By-product is oxygen (O2).
- Earth’s “second atmosphere” came from Earth itself. There were lots of volcanoes, many more than today because Earth’s crust was still forming. The volcanoes released
- steam (H2O, with two hydrogen atoms and one oxygen atom),
- carbon dioxide (CO2, with one carbon atoms and two oxygen atoms),
- ammonia (NH3, with one nitrogen atom and three hydrogen atoms).
- Current Earth: Plants and animals thrive in balance. Plants take in carbon dioxide (CO2) and give off oxygen (O2). Animals take in oxygen (O2) and give off CO2. Burning stuff also gives off CO2.
- Much of the CO2 dissolved into the oceans. Eventually, a simple form of bacteria developed that could live on energy from the Sun and carbon dioxide in the water, producing oxygen as a waste product. Thus, oxygen began to build up in the atmosphere, while the carbon dioxide levels continued to drop. Meanwhile, the ammonia molecules in the atmosphere were broken apart by sunlight, leaving nitrogen and hydrogen. The hydrogen, being the lightest element, rose to the top of the atmosphere and much of it eventually drifted off into space

Now we have Earth’s “third atmosphere,” the one we all know and love—an atmosphere containing enough oxygen for animals, including ourselves, to evolve.
So, Plants and some bacteria use carbon dioxide and give off oxygen, and animals use oxygen and give off carbon-dioxide—how convenient! The atmosphere upon which life depends was created by life itself.
Do you know
Formation of the layered structure in the lithosphere:
- The earth was mostly in a volatile state during its primordial stage.
- Due to the gradual increase in density the temperature inside has increased. As a result of the material inside started getting separated depending on their densities. This process is called differentiation.
- This allowed heavier materials (like iron) to sink towards the centre of the earth and the lighter ones to move towards the surface.
- Due to this earth got divided into layers like the crust (outermost), mantle, outer core, and inner core (innermost).
- From the crust to the core, the density of the material increases.
No comments:
Post a Comment